SOTE - Chemistry Test2
0 of 20 questions completed
Questions:
- 1
- 2
- 3
- 4
- 5
- 6
- 7
- 8
- 9
- 10
- 11
- 12
- 13
- 14
- 15
- 16
- 17
- 18
- 19
- 20
Information
Hello, Dear Ones,
CIS Group provides all registration services for the entrance exams of Hungarian universities for free.
Applicants can send a copy of their passport and email to our email address or our WhatsApp number: 0017788462245.
Our Email:
[email protected]
Please note the following:
This test belongs to Semmelweis Medical University in Hungary.
The test consists of 20 Chemistry questions.
Each correct answer has 5 points and each wrong answer has 1 negative point.
The answer to each question is 60 seconds.
Choose the best word or phrase to fill in the blank.
” Good luck “
با سلام خدمت شما عزیزان
موسسه ما کلیه خدمات ثبت نام امتحانات ورودی دانشگاههای مجارستان را به صورت رایگان انجام می دهد.
متقاضیان می توانند کپی پاسپورت و ایمیل خود را به آدرس ایمیل ما و یا شماره واتساپ ما : 0017788462245 ارسال نمایند.
آدرس ایمیل ما
[email protected]
لطفا به نکات زیر دقت فرمایید:
این آزمون متعلق به دانشگاه علوم پزشکی سملوایز مجارستان می باشد.
آزمون شامل 20 سئوال شیمی می باشد.
هر پاسخ درست 5 امتیاز و هر پاسخ غلط 1 امتیاز منفی دارد.
مدت پاسخگویی به هر سئوال 60 ثانیه می باشد.
“موفق باشید”
You have already completed the Test before. Hence you can not start it again.
Test is loading...
You must sign in or sign up to start the Test.
You have to finish following quiz, to start this Test:
Congratulations!!!" SOTE - Chemistry Test2 "
0 of 20 questions answered correctly
Your time:
Time has elapsed
Your Final Score is : 0
You have attempted : 0
Number of Correct Questions : 0 and scored 0
Number of Incorrect Questions : 0 and Negative marks 0
| Average score |
|
| Your score |
|
-
Chemistry Test
پاسخ های انتخابی : 0
تعداد سوالات صحیح: 0 و امتیاز شما 0
تعداد سوالات غلط : 0 و نمره منفی 0
-
Thank you for taking this test.
” Good Lock “
- 1
- 2
- 3
- 4
- 5
- 6
- 7
- 8
- 9
- 10
- 11
- 12
- 13
- 14
- 15
- 16
- 17
- 18
- 19
- 20
- Answered
- Review
-
Question 1 of 20
1. Question
5 pointsCategory: Chemistry TestWhich substances could be decomposed by chemical reactions?
- water
- sugar
- mercury
- argon
Correct
Incorrect
Unattempted
-
Question 2 of 20
2. Question
5 pointsCategory: Chemistry TestWhat is the volume of 8 g CO2 at STP? The molar mass of CO2 is 44 g/mol.
Correct
Incorrect
Unattempted
-
Question 3 of 20
3. Question
5 pointsCategory: Chemistry TestUnder the symbol of 2SO3 you may understand
- 2 moles of SO3
- 2 molecules of SO3
- 6 moles of O2
- 2 x 6 x 1023 O atoms
Correct
Incorrect
Unattempted
-
Question 4 of 20
4. Question
5 pointsCategory: Chemistry TestA radioactive isotope has a half life of 10 What fraction of the original amount of the isotope remains after 30 days?
Correct
Incorrect
Unattempted
-
Question 5 of 20
5. Question
5 pointsCategory: Chemistry TestWhich main energy shell can accommodate a maximum number of 8 electrons?
Correct
Incorrect
Unattempted
-
Question 6 of 20
6. Question
5 pointsCategory: Chemistry TestAn element has the electron configuration of 1s22s22p63s23p2. The number of valence electrons is
Correct
Incorrect
Unattempted
-
Question 7 of 20
7. Question
5 pointsCategory: Chemistry TestWhich element – shown with its electron dot symbol – gives a compound with nitrate ion that has the formula of X(NO3)2?
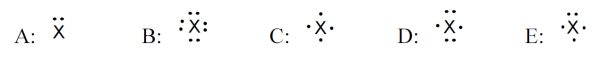 Correct
Correct
Incorrect
Unattempted
-
Question 8 of 20
8. Question
5 pointsCategory: Chemistry TestMagnesium forms an ion with a charge of
Correct
Incorrect
Unattempted
-
Question 9 of 20
9. Question
5 pointsCategory: Chemistry TestWhich molecules contain polar covalent bonds?
- CO2
- CCl4
- F2
- KF
Correct
Incorrect
Unattempted
-
Question 10 of 20
10. Question
5 pointsCategory: Chemistry TestIonic bond is likely to form between the atoms of
- C and Br
- Ca and I
- P and Cl
- O and Na
Correct
Incorrect
Unattempted
-
Question 11 of 20
11. Question
5 pointsCategory: Chemistry TestWhich of the following changes will shift the reaction at equilibrium to the left?
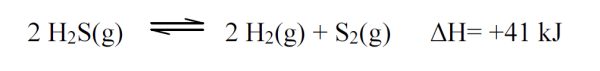
- Increase the concentration of H2S
- Decrease the temperature.
- Increase the pressure.
- Increase the concentration of H2.
Correct
Incorrect
Unattempted
-
Question 12 of 20
12. Question
5 pointsCategory: Chemistry TestWhich solution contains the largest amount of glucose?
Correct
Incorrect
Unattempted
-
Question 13 of 20
13. Question
5 pointsCategory: Chemistry TestChoose the solution with the highest hydronium ion concentration.
Correct
Incorrect
Unattempted
-
Question 14 of 20
14. Question
5 pointsCategory: Chemistry TestThe oxidation number of Mn in MnO ─ ion is
Correct
Incorrect
Unattempted
-
Question 15 of 20
15. Question
5 pointsCategory: Chemistry TestIn the stomach the hydrochloric acid concentration is about 1 M. How many milliliters of the gastric fluid contain 0.5 g of HCl?
The molar mass of HCl is 36.5 g/mol.
Correct
Incorrect
Unattempted
-
Question 16 of 20
16. Question
5 pointsCategory: Chemistry TestYou need to prepare a sodium chloride solution with 10 % m/m If you start from 50 g of NaCl what is the mass of water needed to make the solution?
Correct
Incorrect
Unattempted
-
Question 17 of 20
17. Question
5 pointsCategory: Chemistry TestWhat is/are the missing reactant/s?
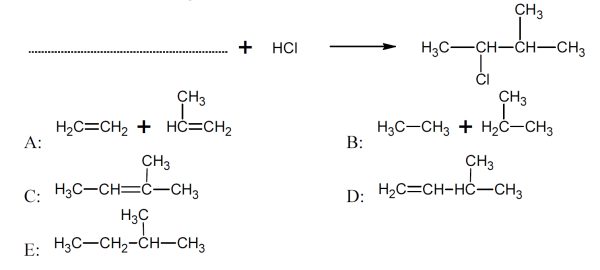 Correct
Correct
Incorrect
Unattempted
-
Question 18 of 20
18. Question
5 pointsCategory: Chemistry TestWhich of the compounds below are amines?
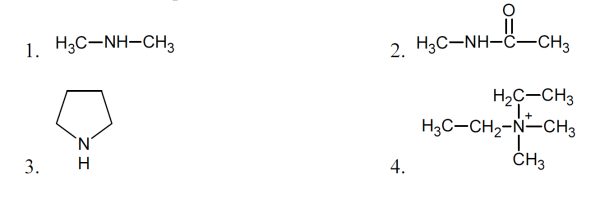 Correct
Correct
Incorrect
Unattempted
-
Question 19 of 20
19. Question
5 pointsCategory: Chemistry TestWhich reactants produce an ester?
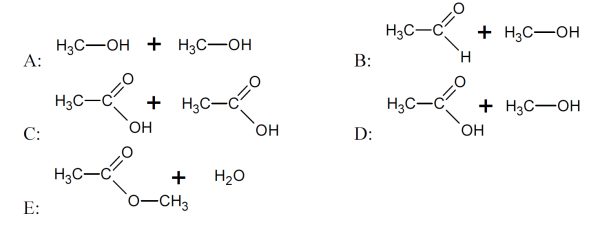 Correct
Correct
Incorrect
Unattempted
-
Question 20 of 20
20. Question
5 pointsCategory: Chemistry TestWhich statement is true for disaccharides?
Correct
Incorrect
Unattempted